Looking for an industrial verification environment for your innovative bio-process technology? Apply to Testa Challenge!
Background
Biological drugs and products are transforming medicine and promise future targeted treatments for a range of conditions. However, for these powerful products to benefit all people in the world, a large number of new technologies and tools that can drastically improve productivity of biological production are needed. New methods, processes and approaches need to be developed in order to accelerate innovation in this area.
Technology or digital projects and companies are invited!
We know that there are countless promising technologies out there. We also know that, as a young technology developing company, it can be a significant threshold to get access to a relevant industrial verification environment. To address this gap, Testa Center now invite to Testa Challenge.
Projects and companies from the technology/digital segments are invited to integrate, test and witness their innovation within a sponsored production workflow at Testa Center. Alongside the production process, participants will be supported by experts and have the possibility to experience their innovation hands on.
We offer
The provided facilities, expertise and fast-tracked application will allow an early evaluation whether the innovation is applicable and successful for the bio-processing industry. The production process will be designed to include approximately five selected technologies and run in Testa Center during October 2020.
What are we looking for?
We welcome all kind of innovative solutions that have the potential to meet the future technology needs within bio-production. Some examples in the area of technology include sensors, separation technology, pumps, etc. and within the digital/data segment, some examples include cloud solutions, VR / AR, AI, data transfer, modeling, automation, smart laboratory tools, etc.
Who can apply?
The call is open for projects and companies from all entities (academic, start-ups, larger companies, and institutes) across Europe. Application opens May 8 2020.
Would you like to have updates?
Register here and you will receive an email as soon as we update the information.
For more information, stay tuned to testacenter.com/testa-challenge and Testa Center at LinkedIn.
Sponsors
About Bio-processing
Traditional drugs, often consisting of chemically synthesized small molecules, are more and more complemented and/or replaced by biological components. The manufacturing of such “biologics”, the bio-production, is a vital part within a company’s product development as it determines if a compound can be produced on a larger scale and reach patients in need.
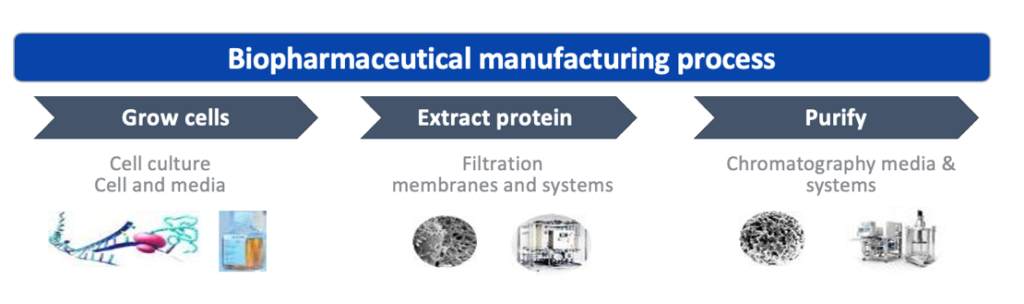
Some examples of biological drugs/products include vaccines, gene therapy and therapeutic proteins that can be designed to target diseases like cancer and many chronic illnesses.
Read more about bio-production, and why is it interesting.
About Testa Center
Testa Center is a non-profit company by Cytiva, formerly GE Healthcare. Co-founded in 2018 by the Swedish agency for innovation, Vinnova, for the growth of the life science industry and its manufacturing capabilities.
Would you like to see the labs? Take a look in at our tour!
We offer businesses and academia globally a modern, pilot-scale testbed for projects and education in production of biological products e.g. monoclonal antibodies, peptides, protein, vaccine and viral vectors (non-GMP, up to Bio-safety level 2 and 500 liter). In our flexible facility, and with some helping hands of our experienced staff, you can perform a wide variety of projects, including scale-up of parts of a process, or a complete process from frozen cell bank vial to purified protein.



